- On this page:
-
 Abstract
Abstract
-
 Publications
Publications
Arming the Immune System to Fight Breast Cancer
Eliminating Cancer Cells
Our research focuses on strengthening the immune system to fight breast cancer, which is the second leading cause of cancer-related deaths among American women.
Abstract
The Northeast Texas region, including Hunt County, is a hot spot for breast cancer incidence, the second leading cause of cancer-related deaths among American women. The introduction of antiestrogen and anti-HER2 therapies has improved the clinical outcomes of breast cancer patients. However, in over half of these patients, cancer will recur and metastasize. A novel strategy is being pursued to restore the ability of the immune system to detect and eliminate cancer cells. Four critical genes that help cancer cells evade the immune system have been identified. Inhibition of these immune-evading genes is expected to be more efficient in eliminating cancer cells than therapies currently available.
Although each cell of a multicellular organism shares the same genetic information with every other cell, the emergence of new information is essential for successful organ development and maintenance throughout the life span of an organism. We will combine hypothesis and discovery-driven approaches to decipher the critical genetic and epigenetic events that coordinate the development and maintenance of organs and how these processes are deregulated in human diseases.
Mechanisms of Mammary Gland Development and Breast Cancer
Mechanisms that govern the maintenance of architecture and differentiated state of organs are deregulated in cancer. Mammary glands are one of the few organs that can undergo repeated cycles of remodeling and morphologic alterations. In each cycle, there are ample opportunities for the complex remodeling mechanisms of the mammary gland to go wrong and cause breast cancer, the second leading cause of cancer-related mortalities in American women. As the initial onset and progression of breast cancer are poorly understood, my laboratory’s long-term goal is to elucidate genetic and epigenetic mechanisms that modulate the plasticity of mammary gland architecture and functions. This may lead to better preventive and therapeutic strategies, a critical aspect of furthering the current success in breast cancer management.
Normal Mammary Acini in Organotypic Assays
G1P3 Induced Hyperplastic Acini
In breast cancer, the basic unit of mammary gland architecture, the acinus, becomes malignant and loses its structural integrity. Using organotypic assays (3D culture), we have discovered that G1P3, one of the first identified interferon (IFN) stimulated genes (ISGs), perturbs the architecture of normal acini by inhibiting the apoptosis of acinar luminal cells to cause hyperplasia. In breast cells, in addition to IFNs, the expression of G1P3 was also induced by estrogen. In agreement with these results, G1P3 was overexpressed in early and advanced stages of breast cancers. More importantly, elevated expression of G1P3 is significantly associated with poor outcomes in ER+ breast cancer, which highlights the clinical relevance of G1P3. Thus, we have uncovered a new arm of signaling used by immuno-endocrine pathways to disrupt cell and tissue architecture, which challenges the dogma of IFNs as the tumor suppressors of the innate immune network. This sparked a desire to understand the functions of G1P3 and other interferon-stimulated genes in modulating the relationship between tissue architecture and cellular functions in normal and diseased conditions. Our current efforts are focused on: 1) How does G1P3 modulate epithelial cell polarity and tissue organization? 2) Role of G1P3 in breast cancer development and progression, and 3) Role of G1P3 in modulating survival functions in immuno-endocrine signaling pathways.
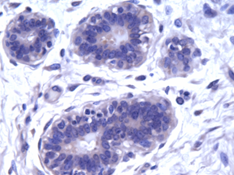
Normal Mammary Gland
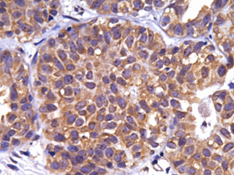
G1P3 Overexpression in Early Stage Breast Cancer


