
Computational Analysis of the Factors that Affect the Outcomes of Hetero Diels Alder Reactions
Our research involves using computational methods to analyze the factors that influence the outcomes of the hetero Diels Alder reaction and predict the outcomes of these reactions.
Abstract
The 2013 Nobel Prize in chemistry was awarded for the efforts of Martin Karplus, Michael Levitt and Arieh Warshel, who laid the foundation for the powerful computational programs that are used today to understand and predict various chemical processes. Computational chemistry is so realistic that it can very accurately predict the outcomes of traditional experiments. In the pharmaceutical industry, computation chemistry is often used to simulate how a drug couples with its target protein in the body, and predict the important interactions and hence assist with the design of new drugs. It is often said that computational modeling programs are just as important for today's chemists as the test tube! One aspect of our research involves using computational to analyze the factors that influence the outcomes of the hetero Diels Alder reaction and to be able to predict the outcomes of these reactions. Ever since the discovery of the Diels-Alder reaction (DA), it has become a cornerstone reaction in organic chemistry for the synthesis of carbon-carbon and carbon-heteroatom bonds. Researchers over the years have been inspired to develop different catalysts to effectively catalyze these reactions. The catalytic asymmetric DA reactions have emerged as a powerful methodology for the stereoselective construction of functionalized six-membered rings with control of regio-, diastereo-, and enantioselectivity. The inverse-electron demand hetero DA (HDA) reactions, which involve the incorporation of heteroatoms, such as oxygen or nitrogen, have given rise to the construction of heterocyclic compounds that are of extreme importance in medicinal chemistry. A major advantage of using the HDA reactions for the synthesis of such compounds is the facile stereospecific introduction of functionalized ring systems with up to four stereocenters in the products.
We have recently studied the effectiveness of a series of proline-substituted derived organocatalysts in promoting the enantioselective outcomes of the HDA reactions of aldehydes with electron-deficient enones.
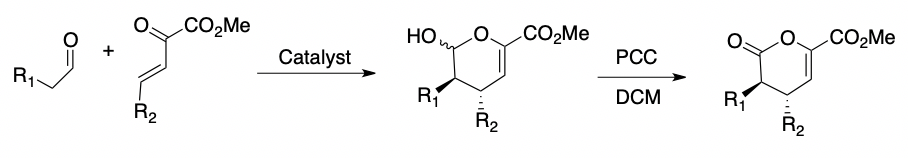
Dr. Allan Headley's research uses computational calculations to predict the outcomes of these reactions. The first phase of this project uses computational calculations to analyze the transition state for the model reaction below.
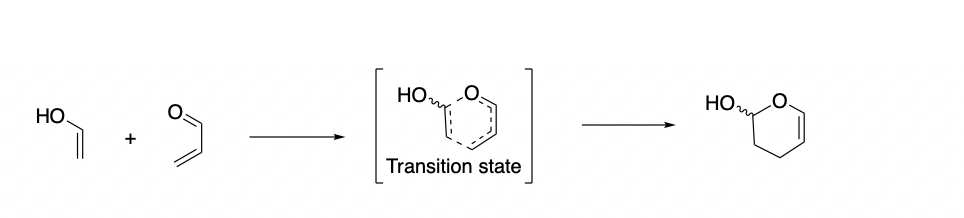
After gaining an understanding of the factors that affect the stability of the transition state for these reaction types, we will utilize the reaction shown below to investigate the effects of different substituents on the diene and dienophile on the regio-, diastereo-, and enantioselectivities of the products.
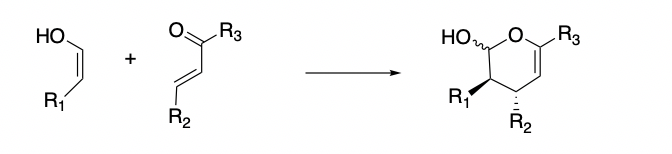
The results obtained via these calculations will be matched against experimental results in order to develop a computational model to predict the outcome of the hetero Diels Alder reactions.


